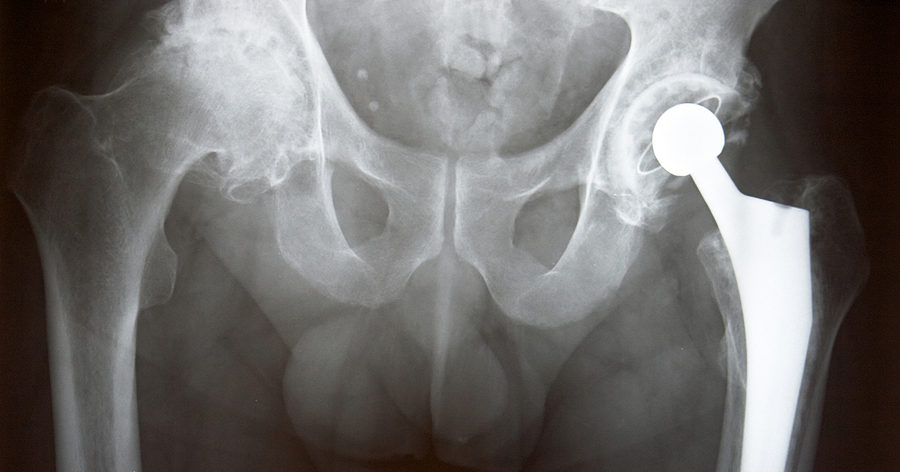
Why File a Biomet Hip Lawsuit?
Plaintiffs in the Biomet hip implant litigation allege that:
• Biomet manufactured and sold a defective hip replacement
• The M2a Magnum hip implant was not properly tested before entering the market
• The company knew or should have known about the potential for the metal-on-metal hips to cause serious injuries
• Biomet failed to adequately warn patients and the medical community about the potential risk of hip implant complications