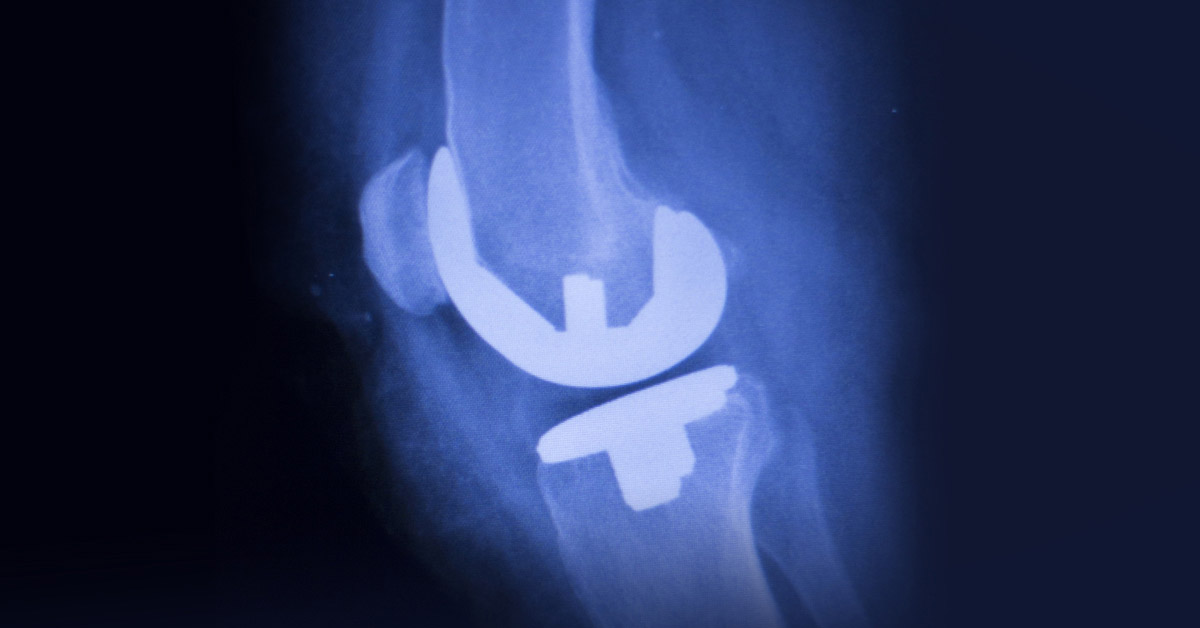
History of the Exactech Knee and Ankle Recall
The issue that prompted the Exactech device recall involves the plastic insert that fits between the device components and acts as cartilage for the replaced knee or ankle joint. These plastic components are packaged in special vacuum bags meant to prevent the plastic insert from wearing prematurely prior to implantation. According to the Exactech recall, the out of specification bags missing this EVOH layer may allow oxygen to diffuse into the plastic insert before it is implanted, thus increasing the risk of device failure and other complications.
Exactech initially announced a recall of its knee and ankle arthroplasty polyethylene inserts in August 2021, after learning about a defect in the packaging. The original recall included devices labeled with an eight-year shelf life not packaged in EVOH bags. In a letter issued to surgeons in February 2022, the company expanded the medical device recall to include all knee and ankle implants packaged in non-conforming vacuum bags regardless of their label or shelf life. Unfortunately, between the recall in August 2021 and the update in February 2022, affected implants were shipped out by the company and implanted by surgeons across the country.